Definitions and Theory
Neurotransmission can be studied in a variety of ways. Neuroanatomical techniques reveal the physical structure of neural pathways. Neurophysiological techniquesilluminate electrical properties of transmission. Neurochemical techniques allowidentification and quantification of molecules involved in neural transmission. In vivo microdialysis is a relatively new neurochemical technique.
Dialysis is the diffusion of soluble molecules across a permeable membrane. The following is a diagram of a concentric dialysis probe.
The concentric probe design is not the only design. There are 3 main types of probe, concentric, U-shaped and transverse, but the principal for the three types is identical. The soluble molecules will flow across the permeable membrane from area of higher concentration to area of lower concentration. The microdialysis probe can, therefore, be used for either sampling or delivery of chemicals. The latter is referred to as retrodialysis. The passage of molecules across the membrane is determined by the surface area of exposed membrane, the concentration gradient the rate of flow through the probe and the type of membrane used. Each membrane has a cut off, measured in Daltons, which can be thought of as a "pore size", and determines the size of soluble molecules which can pass through the membrane.
The probe tip is located in the extracellular space. You can't sample intracellularly or from the synaptic cleft.
Return to Top
Construction
Microdialysis probes are available commercially or they can be manufactured in the lab using dialysis membranes obtained from renal dialysis packs. Typically the external diameter of the membrane is about 250-300µ with a molecular weight cut-off of 20,000 Daltons. The length of the membrane is determined by the area of the brain being probed.
Lawrence(1997) provides the following description of probe construction:
- Insert a wire into the lumen of an individual length of membrane to act as support.
- Insert membrane into 23G cannula. Ensure that membrane is well into cannula (5mm) and secure in place with epoxy.
- Withdraw wire and allow epoxy to dry.
- Cut a 30mm length of polypropylene tubing to fit over 23G cannula and make a hole 10 mm from end with 25G needle. This tubing is placed over the free end of the cannula.
- Trim membrane to desired length and insert fine fused silica capillary tubing through open end of membrane, up through probe and out hole made in polypropylene tubing.
- Seal end of membrane.
Return to Top
Procedure
Before a dialysis probe can be used, it must be tested and calibrated in vitro. The recovery capabilities of probes can be determined by testing the probes in a beaker filled with artificial cerebrospinal fluid and a known concentration of the molecule being studied. After allowing the probe some time to equilibrate samples are collected and analyzed. The recovery ratio of the probe is the concentration of molecule collected in the sample divided by the concentration in the beaker. In actuality the recovery ratio in vivo will be less than recovery ratio in vitro, but calibration of probes is still an important step if only to insure uniformity of construction.
Once the probe has been
calibrated, it is ready to be used experimentally. The
rat is anaethetized and positioned in a stereotaxic instrument. A hole
is drilled through the skull directly over the target area. Care must
be taken to remove the dura mater as well as the skull since the probe
tips are delicate. The probe is then lowered into position using a
manipulator attached to the stereotaxic frame. In chronic procedures,
the probe would be cemented in place, otherwise it is held in place by
the stereotaxic arm for the duration of the experiment. Once the probe
is in position 30- 60 minutes is usually allowed for the probe to
equilibrate before collecting samples or delivering drugs.
Return
to Top
Applications
Microdialysis allows delivery of drugs to or sampling of molecules from the extracellular space without a net loss (or gain) of fluid from the brain. The low flow rate also minimizes damage from hydrostatic pressure. The method can be used to deliver virtually any molecule which will pass through the membrane. Similarly, the technique can be used to measure the extracellular levels of anything that will cross the membrane, provided that a suitable method exists to analyze the sample for the molecule of interest.
Example applications (Lawrence,1997)& Pinel(2000) :
- Identification of
neurotransmitter used by specific neurons.
- Dual dialysis with one probe used for delivery and a second probe used for to measure transmitter release in corresponding terminal field.
- Concomitant dialysis of brain region and peripheral blood vessel to correlate central release of a hormone with blood levels.
- Analysis of concentration of brain levels of a drug following systemic administration.
- Monitoring neurotransmitter levels in behaving animals
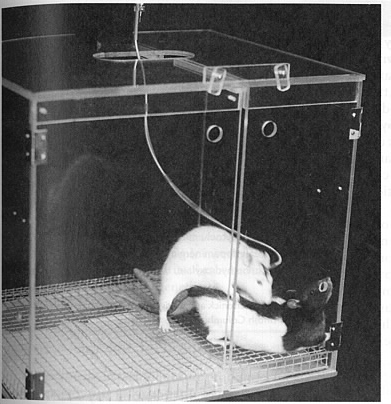
The image at right shows a rat with dialysis probe chronically implanted into the nucleus accumbens. Dopamine levels were found to be elevated in this area when rats were anticipating or engaging in sexual behaviour.
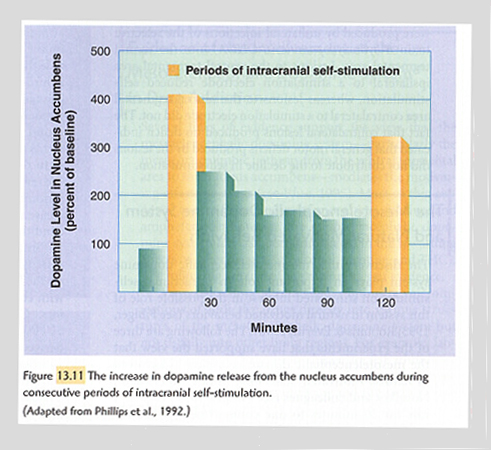
The chart at left shows increased dopamine levels in the nucleus accumbens of rats while engaging in intra-cranial self-stimulation. The stimulating electrode is located in the ventral tegmental area.
Return to Top
24-Mar-2003 02:31 PM
Pitfalls
There are a few pitfalls to the technique of microdialysis:
The probe tips are fairly large and may not be appropriate for small brain nuclei.
Microdialysis only provides you with a sample containing the molecule of interest. You must have a suitable method of analyzing the sample.
The very slow flow rates of fluid through the system result in relatively long sampling times, on the order of 15 to 20 minutes. Such long sampling times make it hard to detect transient changes. Recent modifications to the microdialysis technique are improving the temporal resolution. Some refinements to sampling and analysis methodology have allowed for data collection with a temporal resolution of under 20 seconds.
Return to Top
References
Benveniste,H.and Hüttemeier (1990) Microdialysis- theory and application. Prog. in Neurobiol. 35,195-215.Lawrence, A.J. (1997) Microdialysis in Neuroscience Methods A Guide for Advanced Students Martin,R. ed. Harwood Academic Publishers, Canada
Pinel,J.P.J. (2000) Biopsychology (4th ed.) Allyn & Bacon,Toronto
Steve Milway Last modified:Monday, March 24, 2003 2:32:27 PM