The brain will begin to deteriorate as soon as its blood supply is interupted. The deterioration is rapid, and it is,therefore, important to arrest the deterioration as quickly as possible. The process of preserving the brain is called "fixation".
The two most common ways of fixing the brain are freezing and the use of fixative solutions.Each method has its advantages
.Freezing is a much quicker way of fixing the brain and tends to preserve more of the brains biochemistry, but the tissue is trickier to deal with as it must remain frozen until it is on the microscope slide. There is much blood left in the tissue. Freezing is not appropriate if you intend to do immunocytochemistry. It is good for glycogen phosphorylase staining, AChe staining, Mao staining, and Nissl staining (cresyl violet or thionin). Frozen sections can be mounted on plain glass microscope slides.
The rat brain can be frozen by placing it in the freezer or in the chamber of the cryostat-microtome, but for best results rapid freezing is required. Two common methods of rapidly freezing the brain are dry ice and liquid nitrogen. In our lab we use methyl butane (isopentane) cooled to -72 degrees C. in a ultra cold freezer. A covered jar of methyl butane is placed in the freezer 20-30 min prior to sacrificing the rat. The rat is then anaethetized and decapitated. The brain is quickly removed from the skull and immediately immersed in the cold methyl butane. The brain is frozen within a few seconds. It is then wrapped in aluminium foil and stored in the freezer until sectioning.
The most commonly used fixatives are formalin, glutaraldehyde, and alcohol. A 10% formalin solution is adequate for most purposes, but many stains require specailized perfusions where pH and osmolarity are carefully controlled.
Fixing the brain with fixative solutions takes longer, but the brain is much more resilient and easier to handle. When perfused, most of the blood is removed from the tissue. Fixative Solution are used for immunocytochemistry, HRP staining, and biocytin staining. Nissl stains can also be used on tissue fixed in fixative solutions. Fixative solutions are also used if the tissue is going to embedded in paraffin or plastic and for vibratome sectioning. Tissue fixed with fixative solutions should be mounted on gelatin coated microscope slides if the sections will be processed after the tissue is on the slides.
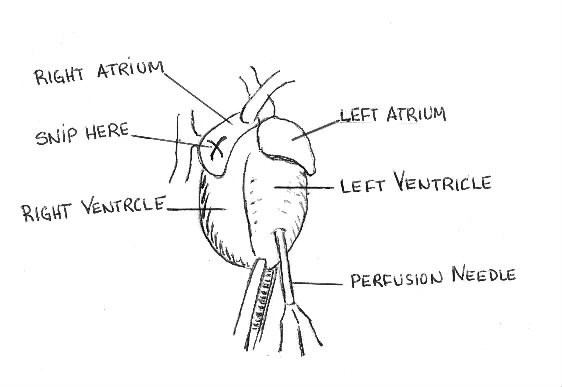
Tissue can be fixed in two ways: by immersion and by perfusion. Immersion is suitable only for small or thin pieces of tissue. In the immersion method the tissue is simply soaked in the fixative solution. The immersion method is often used to fix frozen section that have been mounted on slide. The slides are dipped in formalin or alcohol for a few minutes to fix them after sectioning and staining. Immersion is not a suitable fixation for larger pieces of tissue such as a whole rat brain. The outside of the brain fixes and impedes the penetration of the fixative to the centre of the brain. For whole rat brain, the perfusion method is used. In the perfusion method, the circulatory system is cleared of blood and used to circulate the perfusants uniformly through the brain and body.

Note: The perfusion needle is connected to apump,which pumps the perfusants through the body. The operation of the pump is not covered here. The operation of the pump must be demonstrated.